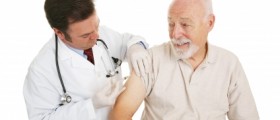
Pneumonia vaccine is a highly effective means of prevention against pneumonia, an inflammatory condition of the lung.
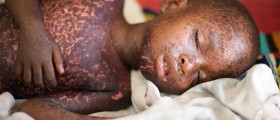
Pneumonia is considered a serious medical condition. It can be caused by many infective agents including bacteria, viruses, fungi, and parasites. In the majority of cases, pneumonia is bacterial in nature. The condition can be triggered by physical or chemical injury. It is estimated that half of a million people are affected by pneumonia each year and that there are 50,000 cases of death due to pneumonia and its complications.
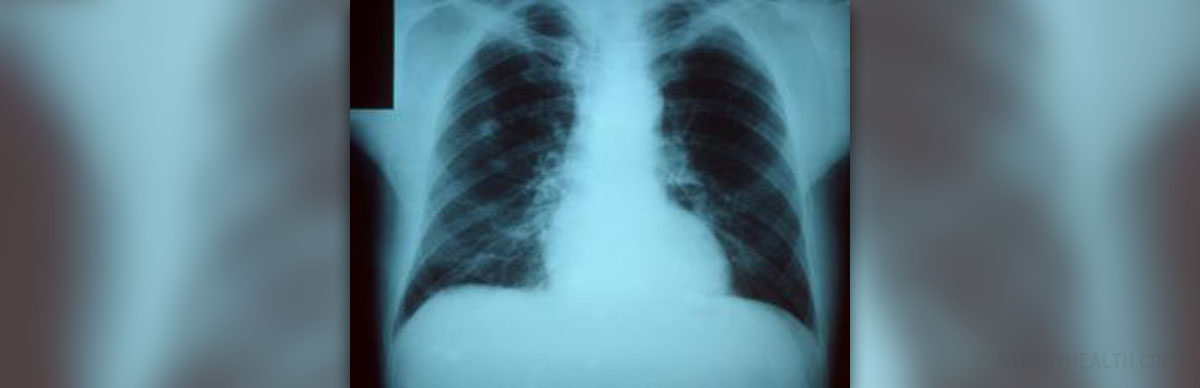
This particular condition mostly affects people over the age of 65 and since older patients already have problems with health and they are more prone to complications of pneumonia. They actually represent the group of patients who can benefit most from the pneumonia vaccine.
The pneumonia vaccine is not able to prevent all types of pneumonia and the disease caused by all infective agents. However, it is highly effective against pneumococcal disease, the most severe form of pneumonia.
- FDA licensed the first pneumococcal conjugate vaccine (PCV7) in 2000. A large clinical trial showed PCV7 reduced invasive disease caused by vaccine serotypes by 97%. Compared to unvaccinated children, children who received PCV7 had 20% fewer episodes of chest X-ray confirmed pneumonia, had 7% fewer episodes of acute otitis media, underwent 20% fewer tympanostomy tube placements.
- In another study, children aged 7 through 71 months received up to 3 PCV13 doses according to age-appropriate immunization schedules. None of the children had previously received a pneumococcal conjugate vaccine. The antibody responses were comparable to those achieved after the 3-dose infant PCV13 series in the U.S. immunogenicity trial with the exception of serotype 1. The IgG geometric mean concentration was lower for serotype 1 among children aged 24 through 71 months.
- Researchers conducted a randomized placebo-controlled trial (CAPiTA trial) in the Netherlands among approximately 85,000 adults 65 years or older from 2008 through 2013. This trial evaluated the clinical benefit of PCV13 in the prevention of pneumococcal pneumonia. The results of the CAPiTA trial demonstrated 46% efficacy against vaccine-type pneumococcal pneumonia, 45% efficacy against vaccine-type non-bacteremic pneumococcal pneumonia, and 75% efficacy against vaccine-type invasive pneumococcal disease (IPD).
- FDA licensed PCV15 and PCV20 in 2021 for use in adults based on studies comparing the serologic response of adults who received either PCV15 or PCV20 to those who received PCV13. These studies showed PCV15 and PCV20 induced antibody levels comparable to those induced by PCV13. The studies also showed PCV15 and PCV20 were safe compared with PCV13. FDA licensed PCV15 in 2022 for use in children 6 weeks through 17 years of age. This was based on clinical trial data showing PCV15 induced antibody levels comparable to those induced by PCV13 and that PCV15 was safe.
- More than 80% of healthy adults who receive PPSV23 develop antibodies against the serotypes contained in the vaccine. This immune response usually occurs within 2 to 3 weeks after vaccination. Older adults and persons with some chronic illnesses or immunodeficiency may not respond as well. Elevated antibody levels persist for at least 5 years in healthy adults but decline more quickly in persons with certain underlying illnesses. Children younger than 2 years of age generally have a poor antibody response to PPSV23.
Who is a Candidate for Pneumonia Vaccine?
Immunocompromised patients are generally prone to infections which means they are also susceptible to pneumonia. They represent only one group of people who can benefit from the pneumonia vaccine. This vaccine does not contain a live culture and can be safely administered to immunocompromised patients.
Furthermore, children who are suffering from cancers (leukemia), kidney failure, or heart conditions are also suitable candidates for the pneumonia vaccine. Their immune system is also jeopardized due to their primary medical conditions.
People over the age of 65 are considered candidates for the pneumonia vaccine as well. But the vaccine can be also administered in people younger than age of 65.
Pneumonia Vaccine Frequency
There is a lot of misunderstanding when it comes to the frequency of pneumonia shots. The actual duration and effectiveness of the vaccine have not been established yet. Even the most prominent medical institutions do not provide sufficient data regarding how often a person is supposed to be vaccinated. In spite of that, there are general recommendations that say that young people are supposed to be vaccinated at an interval of 5-10 years.
This option may not lead to the long-term effectiveness of the vaccine. People above the age of 65 are allowed to have only one shot of the pneumonia vaccine. And finally, younger people may be administered a booster dose. The doctor recommends the frequency of vaccination in the individual case.
Prevention against Pneumonia
Pneumonia is transmitted via respiratory secretions. Coughing, sneezing, and close contact with infected people may allow droplets to enter the nasal or oral cavity of a healthy individual and cause infection.
Contact with infected people is, therefore, forbidden. Washing hands thoroughly in case a person comes in contact with infected saliva or nasal discharge may prevent the spread of the infection. People who are infected are supposed to cover their mouth and nose when sneezing and coughing and to use only disposable tissues.









Your thoughts on this
Loading...