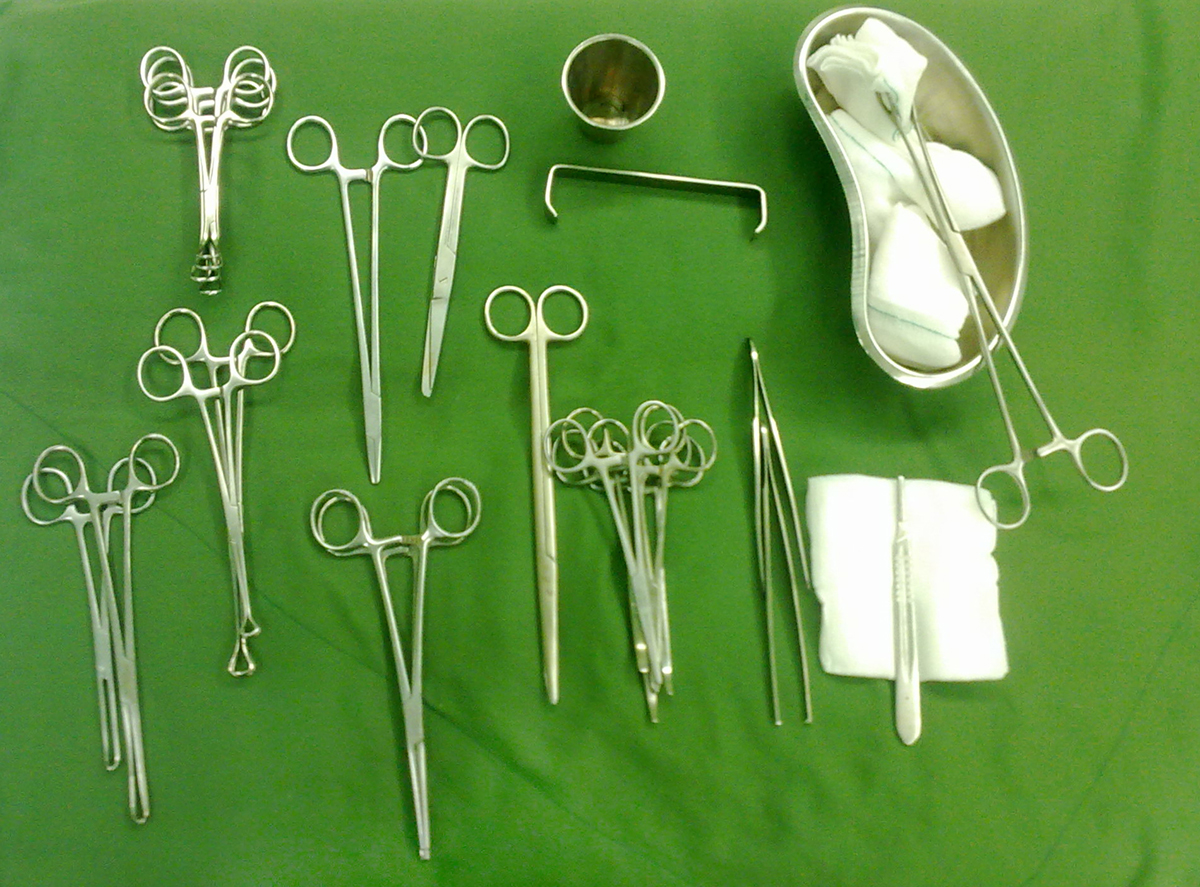
Tubal sterilization is a surgical procedure in which the fallopian tubes are blocked in women who do not plan future pregnancies. Tubal sterilization is a type of permanent birth control and it is also known as tubal ligation or bilateral tubal ligation (BTL). During the operation, the fallopian tubes are cut or tied in order to prevent movement of the egg from the ovary to the uterus as well as to block sperm from reaching the egg. Tubal sterilization is safe and reliable method, 99.5% effective as birth control. Around 700,000 of these procedures are conducted in the United States each year. Tubal sterilizations are often performed after childbirth, in combination with Cesarean section, but can be done at any time in an outpatient clinic.
Tubal Sterilization Procedure
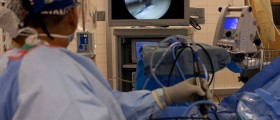
Tubal sterilization is performed under local or general anesthesia. During operation, one or two cuts are made in the abdomen usually under the belly button. A surgeon uses a small telescope on a flexible tube, known as laparoscope and inserts it into the incisions. Through the laparoscope, other instruments can be inserted to conduct the procedure. Then, the fallopian tubes can be burned (coagulated), blocked by small clips or rings, or sealed shut with cautery. After closing the tubes, the instruments are removed and the incisions are closed with stitches. The surgery takes about half an hour and the patient is released home after few hours. Pain relievers can be prescribed if necessary. The patient is advised to perform only light activities several days after the operation. Also, sexual activity can be resumed as soon as a woman feels comfortable. As already mentioned, tubal ligation can be also done during a C-section after childbirth. Tubal sterilization can be performed with two methods. Laparoscopy, also called bipolar laparoscopy, Falope ring and Filshie clip is a method mainly used in non-pregnant women while minilaparotomy is commonly used after childbirth.
The fallopian tubes can be also blocked using a device called The Essure System. The Food and Drug Administration has approved The Essure System as a type of permanent birth control. It is a small metallic implant inserted into the fallopian tube for permanent sterilization. The Essure system doesn’t require incisions but it is inserted through the vagina via uterus into the fallopian tubes. The procedure is done during hysteroscopy. The Essure device works by causing scar tissue over the implant, which blocks the fallopian tubes and prevents sperm from reaching the egg.
Risks
Tubal ligation is associated with several risks. One of them is risk related to general anesthesia as there is a possibility of allergic reaction to anesthetic. Like any surgical procedure, tubal ligation can result in bleeding or infection. Also, there is a slight risk of getting pregnant after the tubal sterilization as well as the risk of ectopic pregnancy. Finally, there is a risk of injury to the organs since instruments are inserted into the abdomen during the operation.








Your thoughts on this
Loading...