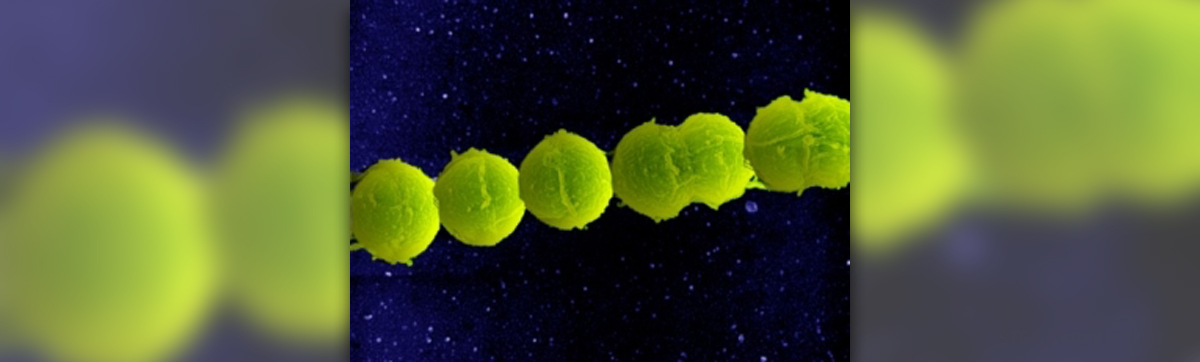
The CDC, Centers for Disease Control and Prevention, is currently working on new recommendations to help the prevention of Group B Strep disease. Though GBS is a relatively common bacterium that can be found in the intestinal tract of up to 40 percent of all adult human beings, it can cause serious complications for babies during and after birth. In fact, GBS disease (which can cause meningitis, pneumonia and sepsis!), is still the leading cause if neonatal deaths, according to the CDC. In the 1970s, the recommendations for GBS treatment were intrapartum antibiotics for all births.
I am sure that there is no need to mention that taking antibiotics where they are not warranted carries its fair share of risks, including in the long term, the development of "superbugs" like MRSA, which are resistant to antibiotics. Perhaps that is one of the reasons why Group B Streptococcus screening and testing was introduced over the last decades. This screening involves a vaginal and rectal swab for women between 35 and 37 weeks gestation. If a woman tests positive for GBS, she can then receive the relevant IV antibiotics during labor. The new CDC guidelines, which are currently being worked on, will include strategies to prevent GBS infection for preterm infants, and more effective methods of detecting GBS in mothers.












Your thoughts on this
Loading...