Primary biliary cirrhosis is a chronic, progressive condition affecting the bile ducts of the liver. The irritation and swelling of these ducts is accompanied by blockage of bile flow. As a result, the obstruction leads to damage to liver cells which eventually causes liver cirrhosis.
Primary Biliary Cirrhosis Causes and Risk Factors
It has not been fully understood why bile ducts get inflamed in the first place. What is estimated is that primary biliary cirrhosis in many cases affects middle-aged women. Obstruction that lasts for a long period of time is responsible for severe damage to the liver and the onset of liver cirrhosis.
This medical condition may be closely related to some autoimmune disorders like celiac disease, Sicca syndrome, hypothyroidism or Raynaud's phenomenon. So people suffering from these conditions are actually at higher risk of developing primary biliary cirrhosis as well.
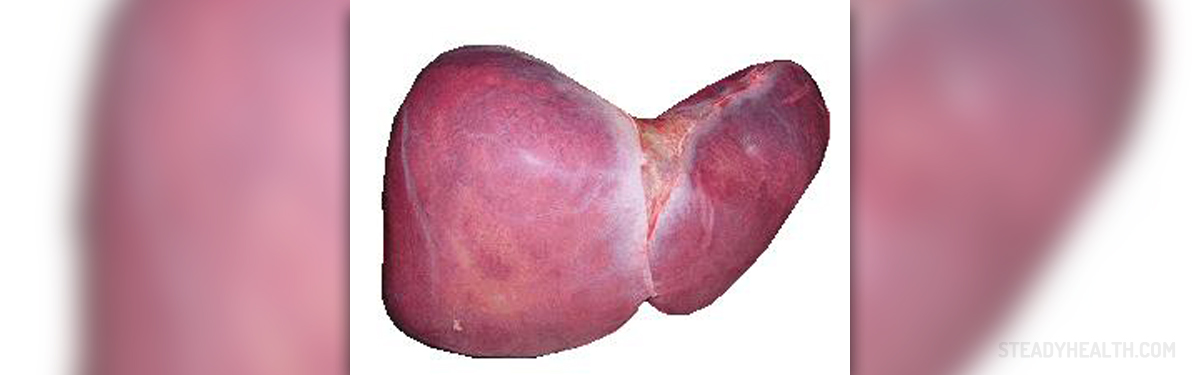
Primary Biliary Cirrhosis Clinical Characteristics
The condition is asymptomatic in around half of all patients at the time of diagnosis. Once the symptoms occur, they gradually intensify.
Abdominal pain is one of common complaints. Furthermore, there are liver enlargement, fatigue, fatty deposits under the skin, fatty stool, itching, jaundice and soft yellow spots on the eyelids.
Diagnosing Primary Biliary Cirrhosis
After performing physical examination and thorough investigating patient's medical history doctors opt for several more tests. They perform tests for liver dysfunction including serum albumin, liver function tests, prothrombin time, serum cholesterol and lipoproteins etc.
More specific tests which can confirm the condition are liver biopsy, conformation of elevated immunoglobulin M and the presence of anti-mitochondrial antibodies.
Primary Biliary Cirrhosis Treatment
The goal of the treatment for primary biliary cirrhosis is to reduce the intensity of symptoms and prevent potential complications.
Cholestyramine is a drug that efficiently deals with itching. Removal of bile is accelerated by ursodeoxycholic acid. By eliminating bile, it cannot accumulate inside the bile ducts and cause inflammation. So this drug actually improves the survival of patients suffering from primary biliary cirrhosis.
Such patients additionally need vitamin replacement therapy (vitamins A, D and K), calcium supplements and only if cirrhosis is severe and is associated with advanced liver failure, one needs to undergo liver transplantation.
- The optimal dosage for UDCA of 13-15 mg/kg per day is the standardized treatment for PBC, as it can postpone its development, improve long-term clinical outcomes, and is extremely safe and well-tolerated. Therefore, reliable identification of so-called treatment non-response to UDCA is very important, not only for selecting PBC patients who could benefit from new therapeutic approaches, but also for discerning those who are at low risk of developing end-stage PBC. The biochemical response to UDCA after 1 year of treatment in PBC has been deemed to be a powerful predictive indicator of long-term clinical outcomes and thus facilitate the rapid recognition of patients requiring novel treatment methods.
- Budesonide is a corticosteroids receptor/pregnane X receptor (PXR) agonist. Treble treatment with budesonide (6 mg/d), UDCA (13-15 mg/kg per day), and mycophenolate mofetil (1.5 g/d) may afford an advantage in non-cirrhotic PBC patients with characteristics of serious illness without biochemical response to UDCA. Combination therapy of budesonide (6 mg/d) and UDCA (15 mg/kg per day) was able to ameliorate the plasma biochemical index of hepatic function and hepatic histology, particularly in PBC patients with hepatic fibrosis (grade?I-III), whereas the treatment effectiveness of UDCA alone was principally on lab results.
- The immunosuppressive agent methotrexate (MTX) has a long history in the treatment of PBC, however little is known about its action mechanisms and roles, if any. MTX was assessed for PBC treatment, which is currently recommended only in patients for whom PBC failed to respond adequately to UDCA and in autoimmune hepatitides/PBC overlap syndromes. PBC patients with characteristics of autoimmune hepatitides should be considered for immunosuppressive therapy, with the therapeutic goal being to attain normal serum aminotransferase levels and histological improvement.
- FXR is the receptor for primary bile acids expressed in enterohepatic tissues, where it regulates bile acid uptake, metabolism, and disposal, and has been considered a significant target for intrahepatic cholestatic illness therapy. Obeticholic acid (OCA) is a semi-synthetic bile acid analogue for 6?-ethyl-chenodeoxycholic acid that is nearly 100-fold more potent than chenodeoxycholic acid (CDCA) and is a powerful, first class alternative FXR agonist derived from primary human bile acid CDCA, the natural endogenous FXR agonist.
- Recurrence of PBC after hepatic transplant has been proven to adversely influence transplant and patient survival. Protective potencies of cyclosporine A (CyA) against PBC recurrence after hepatic transplant have been reported. Changing from tacrolimus to CyA was possible without sequelae, with no patients demonstrating recurrence of PBC. Therefore, CyA might be serviceable for the prevention of PBC recurrence after living-donor hepatic transplant. However, a retrospective multicenter study in Japan showed that although there was no influence on patient survival, original immunosuppression with CyA was considered to be major risk for PBC recurrence after hepatic transplant. However, in subset analysis, switching from tacrolimus to CyA within 12 mo reduced recurrence.
- Fibrates, including bezafibrate and fenofibrate, may be useful for treating asymptomatic patients with PBC who exhibit inappropriate response to UDCA. A nationwide retrospective survey in Japan demonstrated that normalizing serum ALT concentrations with accessional bezafibrate therapy observably reduced the occurrence of hepatic illness-associated clinical signs in symptomless patients, with PBC responding incompletely to UDCA.
- Rituximab, an anti-CD20 monoclonal antibody that selectively depletes B cells, which are precursors of the autoantibody-producing plasmocytes, may be successfully used in autoimmune-mediated hepatic illnesses. Selective depletion of B-cells with rituximab was safe and related to an obvious reduction in autoantibody product, but had limited biochemical effect in PBC patients without optimal biochemical response to UDCA.
- Mesenchymal stem cell (MSC) transplantation is considered to be safe, and has been diffusely tested in autoimmune hepatic disease clinical trials with encouraging results. MSC transplantation could modulate the systemic immune response and promote recovery in hepatic inflammation of PBC.
- At present, liver transplantation (LT) is still a lifesaving approach with outstanding results for end-stage PBC patients. Although the 15 year survival of PBC patients was confirmed as 52.6% after LT, regrettably, the recurrent rates of PBC were 21%-37% and 43% at 10 and 15 years after LT, respectively. Though there is still no specific treatment for recurrent PBC (rPBC), cyclosporine A and UDCA may be useful for the prevention of rPBC after LT.
Primary Biliary Cirrhosis Prognosis and Potential Complications
Patients who are not treated inevitably end up with liver failure. Still, this complication may also affect patients who have been properly treated. It is estimated that around 25% of all patients who have been suffering from primary biliary cirrhosis for 10 years are going to develop liver failure.
The disease is associated with certain complications which generally develop when the disease reaches advanced stages. They include progressive cirrhosis accompanied by liver failure, bleeding, damage to the brain in a form of encephalopathy, fluid/electrolyte imbalance, malabsorption/malnutrition, kidney failure and osteomalacia.












Your thoughts on this
Loading...