Occurrence of Radon
Radon is a radioactive noble gas which does not have anytaste, odor or color and it occurs in its natural form as the decay product ofradium. When it comes to gases, radon is one of the densest substances known toman and it is commonly associated with health hazards due to its pronouncedradioactivity. It has many isotopes, but the most stable one is 222Rn which ischaracterized by a half-life of 3.8 days. Uranium has been present on earthsince forever and its half-life is 4.5 billion years. Its normal radioactivedecay chain involves the formation of radon. In most cases of public exposureto ionizing radiation, radon is the single largest contributor to individualbackground radiation dozes as it accumulates in buildings, basements andattics. Radon can also be found in hot springs and spring waters. According tocertain statistics, high concentrations of radon may be related to lung cancer.Radon is the second on the list of the contributing factors, right behindcigarette smoking. Certain geographic areas are characterized by heightenedconcentrations of radon, so therefore this gas is considered as a rathersignificant indoor air contaminant. The concentration of radon in the atmosphereis measured in becquerels per cubic meter. This unit is an SI derived one, soit is a standardized one. As far as radon is concerned, typical exposureinclude 10 to 20 Bq per cubic meter outdoors and approximately 100 Bq per cubicmeter indoors. It is very important for the mining industry to constantlymonitor the working level index and compare it to the cumulative exposure overa number of working level months. The working level index is measured in joulesper cubic meter of air, while the cumulative exposure in working level monthscan be measured by another SI standardized unit called joule hours per cubicmeter. A single WLM of cumulative exposure is approximately the same asspending a year in any atmosphere characterized by radon concentration of 230Bq per cubic meter. Once radon gets released into the air it gradually decaysover time to different type of radioisotopes. The concentrations of radon inour natural environments are commonly very low, but still, those concentrationsmay vary greatly from place to place. The concentrations are the lowest overthe oceans, and they are the highest in poorly ventilated dwellings, caves andaerated mines. Radon can be found emanating from the building materials andfrom the ground because there are traces of thorium and uranium layingeverywhere around the world. Radon is a very rare gas so it migrates freelythrough fragmented soils and faults and it is not uncommon for it to getaccumulated in water or caves. The concentrations of radon in nature may alsovary greatly depending on the weather conditions and the actual season. Due tothe fact that it has very similar pressure and temperature curve as propane,radon may also sometimes be found in petroleum. The highest averageconcentrations of radon in the United States can be found in Iowa andPennsylvania. There are also certain pieces of gold jewelry which arecontaminated by radon.
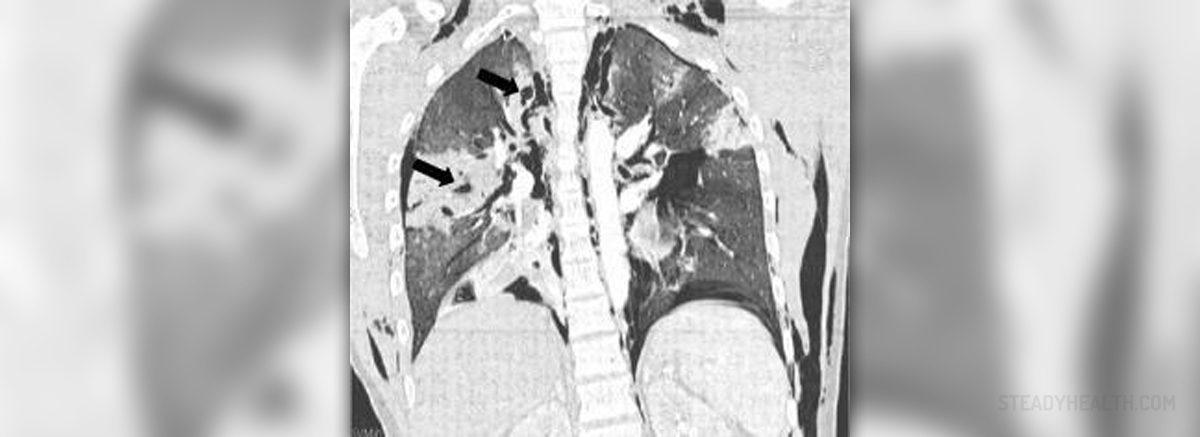
According to various scientific studies, cumulative exposureto radon may be associated with the development of lung cancer in some cases.This presents an extremely high risk of jeopardizing the overall health,especially for hard rock minors. This is why all mines need to be properly ventilatedin order to avoid the accumulation of radon in excessive amounts.
The International Agency for Research on Cancer hasclassified radon-222 as a substance which causes cancer in humans. The WorldHealth Organization has also recommended a reference level of exposure which is100 Bq per cubic meter. They also urged strengthening of the radon measurementand mitigations programs everywhere around the world, along with thedevelopment of building codes characterized by prevention measures. Numerousstudies from everywhere around the planet have shown that the lung cancer rateis on the constant rise, and it is often associated with exposure to radon andits numerous different types of decay products. Exposure to radon usuallyoccurs due to inhalation and the very exposure is considered to be of indirectnature. Radon in itself does not cause any unwanted medical conditions. Variousdifferent types of radioactive products which get produced during the decay ofradon are actually what is harmful to the health of humans. High concentrationsof radon are only associated with a cancer of the lungs, as they are theprimary targets of the toxicity. The most hazardous of all different types ofproducts released during the decay of radon are polonium-214 and polonium-218. Oncethese harmful isotopes reach the lungs of a human being, they trigger damage,DNA breaks and an intense production of the harmful free radicals.















Your thoughts on this
Loading...